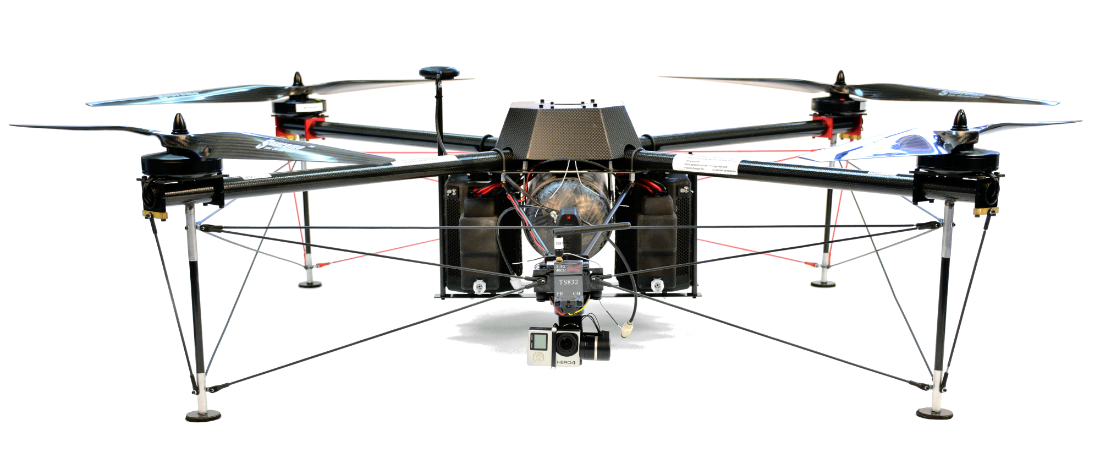
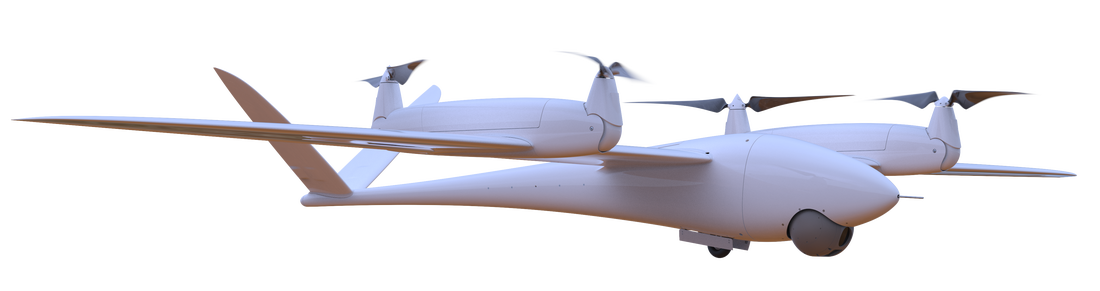
LONGEST MULTIROTOR FLIGHT ENDURANCE IN THE WORLD
2 HR FLIGHT ENDURANCE WITH 1 KG PAYLOAD

VERTICAL AND HORIZONTAL FLIGHT
UP TO 8 HOUR ENDURANCE
POWERED BY FUEL CELL
(COMING SOON)

ENERGY & POWER ANYWHERE-CONTINUOUSLY (APU)
DESIGNED SPECIFICALLY FOR FIELD APPLICATIONS
ENERGYOR CAN DELIVER FUEL CELL SYTEMS THAT ARE TAILORED TO YOUR SPECIFIC REQUIREMENTS

DESIGNED SPECIFICALLY TO DELIVER EXTENDED FLIGHT ENDURANCE.
3 HR 45 MN ENDURANCE (2014)
Watch EnergyOr's H2QUAD 1000 drone fly in a french military operation